Transvaginal OPU and IVFEP Part 2: future of bovine genetics?
John Dawson, in the second part of his article, describes three steps to processing and maintaining the quality of oocytes.
In the first part of this article the author discussed the apparatus needed to collect oocytes and the process involved to keep them in premium quality as they are transported to the laboratory. This article describes how they are processed and steps needed to maintain their quality.
Three steps to good quality embryo production
Figure 1. Ablation of the dominant follicle. The hyperechoic line of the needle can be seen in the lumen of the large follicle on the top right of the image.
Successful production of good quality embryos using ovum pickup (OPU) and in vitro fertilisation and embryo production (IVFEP) depends on three things:
- optimising donor preparation
- perfected OPU technique
- excellent oocyte handling and laboratory process
Step 1: donor preparation
All donors need to be in good health and on a well-balanced, nutritious diet. One of the most important things – especially in older donors – is a diet that does not create weight gain or, perhaps more importantly, fat deposits, as these can seriously hamper the control for collection technique. Donors are usually housed at centres where the collection equipment is located. Here, the care, including the nutritional input of the donor, can be optimised and consistent.
Oocytes collected within cumulus-oocyte complexes (COCs) during the progesterone phase can be of significantly lower quality than those collected from the ovaries without a corpus luteum (CL) in the oestral phase. This can be explained by the higher numbers of follicles that have become atretic or are in the early development stage, during the progesterone phase, compared to the oestral phase. To overcome this quality issue, follicle-stimulating hormone (FSH) programmes, combined with coasting, are used to mature the follicles and COCs – increasing their quality and harvestability.

Follicle-stimulating hormone programmes
Before the OPU process starts, donors are primed with FSH. Multiple injections of FSH for superstimulation of the ovaries was first used in 2002 (Blondin et al) and the dose rate and protocol have been modified to superovulate the ovaries for the maximum production of oocytes.
Figure 2. The ovum pickup process is underway. The needle is seen at the bottom of the ovary retrieving a cumulus-oocyte complex.
FSH stimulates the maturation of the oocytes and, ultimately, increases the yield of embryos. It does not raise the number of COCs collected, but does increase conversion of the COCs to a viable embryo with a better chance of subsequent survival after transfer.
The donors are programmed with FSH and, in many cases, oocytes are collected every two weeks. FSH is given over a three-day, twice-daily dose regime to maximise COC production. The dose can vary between donors and is dependent on their previous response and size of follicles.
Coasting
Coasting is the term used to describe the variable waiting period between the last FSH injection and the point of COC collection, and varies depending on the individual donor’s performance. This waiting time is very important and influences the number and quality of oocytes, and subsequent embryo numbers developing to the transferable stage. The coasting period can range from 20 to 54 hours, but, typically, is approximately 38 to 46 hours. At the optimum point, two-thirds of the follicles are between 8mm to 10mm in diameter; the remaining third are a split between larger and smaller diameters.

Corpus luteum
Studies show the negative effect of the CL on the developmental competence of bovine oocyte depends on the follicle size. Therefore, oocytes originating from large-grown follicles are not influenced by the negative effects of the CL as much as those originating from small and medium follicles. Consequently, if FSH is used alone without CL regression, the resultant larger follicles can prove better quality and a higher percentage develop to blastocysts after the laboratory process (Karami Shabankareh et al, 2015).
Figure 3. A suction unit can be calibrated to the correct flow rate for safer collection of cumulus oocyte complexes.
Progesterone-releasing devices are used by some teams in conjunction with the FSH programme to prevent the cow cycling. However, many teams suggest supplementary progesterone is not required to improve the efficiency of OPU in cattle, unlike sheep.
Dominant follicle elimination
Eliminating dominant follicles at the start of the FSH programme can improve the subsequent FSH-influenced development of the follicular crop. The dominant follicles are eliminated via different methods. Ablation of the dominant follicle can be performed by using the OPU apparatus (Figure 1). Hormonal programmes using gonadotropin-releasing hormone and luteinizing hormone can also be used.


Cumulus-oocyte complex yield per collection
The number of COCs recovered per OPU varies from cow to cow; however, cows and heifers have repeatable numbers of follicular structures in their follicular waves. Females with bigger ovaries, and more visible follicles, are likely to be more fertile and yield more embryos with normal embryo transfer or more COCs with OPU. The number of COCs collected using OPU is dependent on the individual donor and has as variable an embryo production as conventional in vivo embryo collection procedures. Thus, embryo production is donor-dependent, whether you use OPU or conventional in vivo collection methods.
Figure 4. Cumulus-oocyte complexes found after a collection.
COC numbers collected from some donors will be as low as 3 to 4, while, from a productive donor, it can be more than 20. Embryo production from collected COCs varies depending on the skill of the OPU operator and the quality of the laboratory culture, maturation and conception of the COCs, but can be as high as 60% of the COCs collected.
Step 2: collection technique
The ultrasound probe and handle with its puncture needle and aspiration guide needs precise alignment with the follicles, as well as optimal suction pressures to ensure collection without spillage or damage of the oocyte. The sensitivity of the ultrasound equipment is extremely important for maximising the number and quality of oocytes recovered.


Ovum pickup collection method
The left hand is inserted into the rectum and the right hand introduces the transducer into the vulva until the convex probe is positioned against the anterior vaginal wall. The transducer is tilted to the side corresponding to the ovary being aspirated. The left hand elevates the ovary to the abdominal side of the anterior vaginal wall, aligning it with the transducer so it appears on the ultrasound image.
The first follicle is presented to the area where the needle will puncture the vaginal wall. Alignment of consecutive follicles is done while the needle remains in the ovary – this is difficult and requires a high level of skill.
Figure 5. Typical incubator for transportation of cumulus-oocyte complexes to the laboratory.
All large follicles situated on the cortex of the ovary are collected. With every additional follicle punctured and collected, the ovary becomes smaller, making holding and positioning for the subsequent follicles more difficult. It is normal to leave the largest follicle for last, as this makes holding the ovary easier (Figure 2).
The follicles are best approached avoiding the stroma and are collected under the ovarian capsule, and around the periphery of the CL. The CL is avoided at all costs. Regular withdrawal and realignment is required for two reasons: firstly, because collection lines need to be cleared regularly (the author suggests after five follicles), and, secondly, the procedure will help restrict ovary trauma.
The suction unit is calibrated before starting the OPU procedure (Figure 3). It should be calibrated to a flow rate of 7ml to 10ml per 30 seconds. If it appears the oocytes are being denuded, the suction pressure is turned down. It is important the vacuum is started before entering the follicle.
Between 5 and 10 follicles are aspirated. The apparatus is then removed and the needle and tubing washed through with collection media containing heparin. Media ingredients are very specific and have been developed and refined to maintain maximum COC quality. This is done because the aspiration equipment will get blocked if too many follicles are aspirated in one puncture. Heparin is added to the media to prevent clotting.
It takes approximately 30 seconds for an experienced operator to aspirate 7 to 10 follicles (Figure 4).


Step 3: culture, fertilisation and embryo maturation
The COCs are transferred from the collection media in to maturation media as soon as possible. This can be done at the on-site laboratory or collection site before being transported to the laboratory, which can be, in some cases, hundreds of miles away.
Care should be taken to transfer COCs from the collection media with as little media as possible. If too much collection media is transferred it will dilute the maturation media and impair the COCs’ development.
Figure 6. A mixture of good and poor-quality cumulus-oocyte complexes (COC) aids development. Good quality COC can be seen with a thick layer of cumulous cells; poorer quality COC have a thin layer of cumulus cells.
The COCs are carried in the transport incubator and matured in the maturation media during the first 24 hours. Maturation takes place during transportation as the COCs are kept in it during transit (Figure 5). Matured COCs are transported in groups. They benefit from being in groups as the cumulus cells produce chemicals that aid the maturation process and future embryo development. Therefore, mixing good and poor-quality COCs enhance each other’s quality (Figure 6).
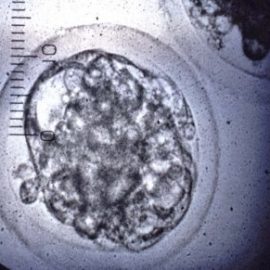
Semen is then introduced to the oocytes for fertilisation to take place. Once fertilisation has been successful, the embryo is matured in media for a further seven days, reaching the late morula/early blastocyst stage of embryo development (Figure 7). The seven-day embryo has approximately 145 cells arranged in a tight cell mass within the intact zona pellucida. These seven-day embryos are either transferred fresh into a synchronised recipient or can be frozen for future transfer.
Successful pregnancies and calves born are achieved with the utmost care and attention given to every step of the production process.
Alternative uses for the ovum pickup unit
Treatment of cystic ovarian disease
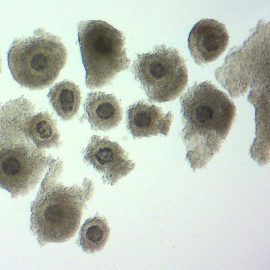

Figure 7. A good quality early blastocyst embryo for transfer or freezing.
The OPU unit can be used to physically eliminate ovarian cysts. This form of cystic treatment is very effective – especially for cysts that have not responded to other forms of treatment. This procedure has been successfully carried out repeatedly in some cows.
Biopsy of the ovary and uterus
Some reproductive examinations reveal unusual structures on the ovary and around
the reproductive tract. These can be biopsied with the OPU needle and sent to a pathologist to be evaluated.
The future
OPU, along with IVFEP, provide the starting point for a generation of reproductive material for a number of advanced reproduction techniques, including sperm microinjection and nuclear transfer. It has been used while researching the ovarian function. Follicular dynamic manipulation helps us to understand and modify oestrus synchronisation and improve treatment regimes in reproductive problems.
The economic climate in the UK challenges the viability of OPU/IVFEP, but it remains a successful method for genetic improvement and will be used by elite producers to enhance their superior genetic lines. Looking ahead, this technology could be used to easily generate large numbers of embryos of a given genomic score to establish new elite herds – either in their country of origin or abroad. In several other countries, commercial in vitro fertilisation facilities are already being employed by embryo transfer vets and bovine genetic companies.
References
- Blondin P, Bousquet D, Twagiramungu H, Barnes F and Sirard MA (2002). Manipulation of follicular development to produce developmentally competent bovine oocytes, Biology of Reproduction 66(1): 38-43.
- Bols PE, Leroy JL, Vanholder T and van Soom A (2004). A comparison of a mechanical sector and a linear array transducer for ultrasound-guided transvaginal oocyte retrieval (OPU) in the cow, Theriogenology 62(5): 906-914.
- Brackett BG, Bousquet D, Boice ML, Donawick WJ, Evans JF and Dressel MA (1982). Normal development following in vitro fertilisation in the cow, Biology of Reproduction 27(1): 147-158.
- Karami Shabankareh H, Shahsavari MH, Hajarian H and Moghaddam G (2015). In vitro developmental competence of bovine oocytes: effect of corpus luteum and follicle size, Iran Journal of Reproductive Medicine 13(10): 615-622.
- Pieterse MC, Kappen KA, Kruip TA and Taverne MA (1988). Aspiration of bovine oocytes during tranvaginal ultrasound scanning of the ovaries, Theriogenology 30(4): 751-762.